Safeguarding the Supply Chain: The Role of Medical Products Regulation
By Esther Were, Senior Policy and Training Manager, and Kate Kikule, Senior Principal Technical Advisor – Pharmaceutical Regulatory Systems Strengthening, Management Sciences for Health
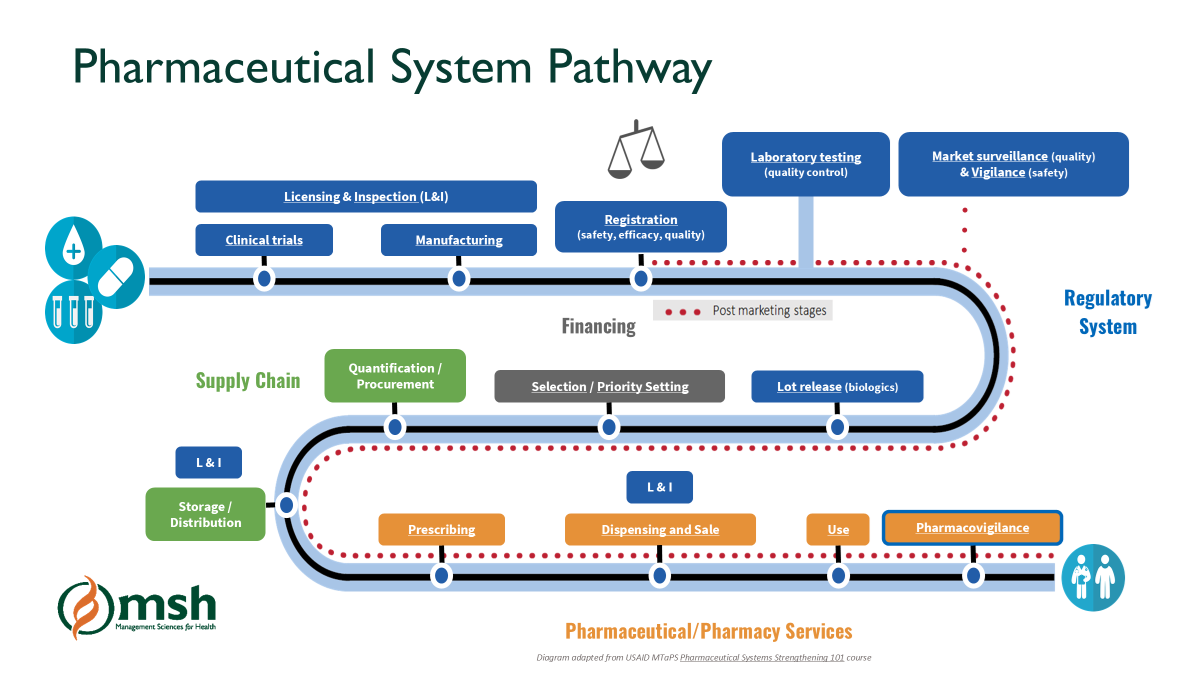
Diagram adapted from USAID Pharmaceuticals Systems Strengthening 101 course
The supply chain for medical products, which includes medicines, vaccines and medical devices, is a complex system that involves delivering products from the manufacturer to the end-user through a distribution channel that encompasses manufacturers, wholesalers, importers, distributors, and retailers. The supply chain system must ensure that the products reaching patients are safe, effective, and quality assured; however, substandard and falsified (SF) medical products continue to circulate in the market, especially in low- and middle-income countries, posing a significant challenge to the health system. SF products pose severe risks for public health, as they do not adhere to the necessary quality, safety, and efficacy standards, leading to treatment failures, drug resistance, and sometimes death.
Regulation plays a vital role in safeguarding the medical supply chain from SF products. By enforcing strict regulatory measures, countries can prevent SF products from entering the legal market, identify and deal with illegal markets, and build a system of accountability among manufacturers and distributors. Through its regulatory systems strengthening work in Africa and Asia, the USAID Medicines, Technologies, and Pharmaceutical Services (MTaPS) Program, led by Management Sciences for Health (MSH), has supported supply chain security by improving pre- and post-registration activities, and strengthening reliance and harmonization efforts in countries and across regions.
Pre- and Post-Registration Activities: How Regulation Impacts the Supply Chain
Regulatory activities can be divided into two primary categories: pre-market and post-market activities. Both are essential in ensuring supply chain security and mitigating the circulation of SF products.
The first line of defense against SF products begins with stringent pre-market activities. These include the product registration process, regulatory inspections, licensing of establishments involved in the production, distribution of medical products, and clinical trials oversight.
Pre-market activities:

500 Pharmacists and 350 Wholesale owners trained in Good Pharmacy Practices (GPP) and GSDP in Nepal. Photo Credit: Aastha Rawal, MTaPS Nepal
Product registration ensures only quality-assured medicines enter the market. However, in some countries, registration systems are not effective and require capacity enhancement and streamlining of processes. In Rwanda, MTaPS worked with Rwanda Food and Drug Authority (FDA) to streamline the registration system by establishing clear registration regulations, guidelines, and procedures; building the capacity of assessors on product dossier evaluation, including such complex substances as biologics and vaccines; ensuring the clearance of dossiers’ backlogs awaiting evaluation; automating the registration system; and enabling reliance on WHO prequalification results and well-resourced national regulatory authorities to improve efficiency and prevent SF products from entering the supply chain.
Regulatory inspections uphold Good Manufacturing Practices (GMP) and Good Storage and Distribution Practices (GSDP) and reduce risks from unlicensed or non-compliant facilities. In Bangladesh, MTaPS introduced an electronic inspection system for drug outlets, streamlining compliance checks and improving the integrity of the supply chain in a more transparent and efficient manner. Training in GMP for manufacturers in India improved their knowledge in GMP for production and supply of quality-assured medicines.

Certification of Medicine Inspectors in inspection of medicines referring to the National Medicines Register at ports of entry in DRC. Photo credit: MTaPS
Clinical trials oversight has become more critical and complex as a result of patient-centered and decentralized approaches. Clinical trials are the gold standard for testing the safety and efficacy of medicines in humans prior to marketing authorization. There is increasing complexity in designing and conducting clinical trials, as well as the need for shortening the time to license medical products. Conducting clinical trials is also changing due to technological advancements such as artificial intelligence and computerization of systems. MTaPS facilitated the creation and advancement of clinical trial departments at the Rwanda FDA and Nepal’s Department of Drug Administration. These regulatory authorities can now monitor clinical trials in their jurisdiction and assure the safety of trial participants and the authenticity of the results generated.
Post-market activities:
Once products are on the market, regulatory authorities must continue to monitor their safety and quality through post-marketing surveillance. Post-market activities are essential for detecting and removing SF products that may have entered the supply chain.
Pharmacovigilance (PV), which monitors the effects of medical products after their release to the market, is crucial for identifying any potential safety issues. PV systems detect post-market adverse events and provide mitigating actions, such as withdrawal of the product, a change in labeling information, reclassification of the medicine for better control of usage, and identifying medication errors and potential SF products. MTaPS supported the regulatory authorities in Cameroon, Rwanda and Mozambique (i.e. Directorate of Pharmacy Services, the FDA and Autoridade Nacional Reguladora de Medicamento (ANARME), respectively) to establish effective PV systems to monitor medicine safety. In Kenya, MTaPS supported the Pharmacy and Poisons Board to create a mobile app to report adverse drug reactions and SF products, improving surveillance and safety monitoring. Countries at maturity level 3, such as Tanzania and Ghana, have strong PV systems in place that encourage healthcare providers and patients to report adverse reactions to medical products. This allows regulatory agencies to quickly identify any adverse events that might also be due to use of SF products.
Market surveillance and control measures identify and remove SF products in circulation. In Mozambique, MTaPS helped ANARME draft regulations and develop control measures, strengthening the national response to SF products and securing the distribution network. Regular inspections of pharmacies, distributors, and healthcare facilities help ensure that products on the market meet regulatory standards. Rwanda and Mozambique have been particularly proactive in conducting market surveillance to detect SF products. In Rwanda, the Rwanda FDA has conducted routine inspections that have successfully identified and removed SF medicines from the market. Control measures allow for the recall, appropriate storage, and disposal of products found to be substandard or falsified. Regulatory agencies in Tanzania and Nigeria have implemented strict control measures to maintain the supply chain’s security by ensuring that unsafe products are swiftly removed from circulation.
Reliance and Harmonization
Reliance and harmonization are key strategies for improving the efficiency and effectiveness of regulatory systems. Reliance refers to the practice of one regulatory authority using the assessments or decisions of another trusted authority to inform its own decisions. This approach reduces duplication of efforts and accelerates the approval process for medical products.
In Africa, the East African Community (EAC) has implemented a regional reliance model, where member states rely on the regulatory decisions of their neighbors. This approach has improved regulatory efficiency across the region and has helped to combat the circulation of SF products by creating a unified regulatory front. Similarly, in Asia, countries participating in the Association of Southeast Asian Nations (ASEAN) Mutual Recognition Arrangement (MRA) rely on the regulatory decisions of other member states, which has enhanced regulatory efficiency and reduced the circulation of SF products.
Harmonization efforts, as seen with the African Medicines Regulatory Harmonization Initiative (AMRHI) and ASEAN’s pharmaceutical harmonization initiatives, provide a framework for countries to adopt consistent regulatory standards, which helps to ensure that SF products cannot easily cross borders. Harmonization reduces duplication by aligning regulatory standards across countries. Regulatory harmonization is a strategic approach to creating uniform standards across different countries and regions, allowing for more efficient management of medical products. In a globalized world, medical products often move across borders, making it crucial to have harmonized regulations that can help prevent SF products from infiltrating different supply chains.
Harmonization efforts are evident in Africa, with initiatives like the AMRHI, which aims to standardize the regulation of medicines across the continent. Similarly, the ASEAN Pharmaceutical Product Working Group harmonizes pharmaceutical regulations across Southeast Asia. Both initiatives play a critical role in preventing SF products from entering these regions by ensuring that regulatory standards are uniformly applied. MTaPS has supported the AMRHI, which enables regional cooperation and shared regulatory decisions, helping countries block SF products at entry points. In addition, MTaPS provided technical assistance to ASEAN to develop capacity for members in medicines and vaccines registration.

South Eastern Asian Regulatory Network (SEARN) members. Photo credit: SEARN
Call to Action
To effectively address the circulation of SF medical products worldwide, we must prioritize creating strong regulatory systems. Policymakers and regulatory authorities must enhance legal and regulatory frameworks, increase stakeholder engagement, and build technical capacity to ensure that regulations are not only in place but actively enforced. Local manufacturers should commit to regulatory compliance and quality assurance practices to foster trust within the supply chain. Consumers must be encouraged to report any concerns regarding product safety and quality, while other stakeholders, including donors and civil society, should advocate for investments in the safety and quality of medical products. The growing emphasis on regulatory reliance and harmonization further strengthens the global response to SF products by improving the efficiency and consistency of regulatory frameworks. By continuing to invest in regulatory systems, countries in Africa and Asia can better secure their medical products supply chains and protect public health from the dangers posed by SF products. Together, through sustained collaboration and commitment, we can strengthen the integrity of our medical products supply chains across the globe and safeguard public health for all.