Medical products are intended to treat and make people feel better. But sometimes, they can also cause harm. Adverse effects of medicines, particularly those that are unexpected by providers and/or by patients, are a source of concern as they can negatively affect patients and undermine trust in the healthcare delivery systems. The concern for causing harm is heightened in case of new medicines and vaccines as they enter markets based on limited data from clinical trials conducted with small, select population groups.
Establishing pharmacovigilance (PV) – both passive (i.e., voluntary) and active (e.g., cohort event monitoring) – once a product is on the market is critical for determining the true safety and efficacy profile of a product in a given population, including identification of rare effects not necessarily seen in clinical trials. This role lies with the national medicines regulatory authority and requires participation and awareness across a wide spectrum of actors – doctors, nurses, pharmacists, and other healthcare providers, consumers, industry, and public health programs (e.g., national TB, HIV and malaria programs) – for reporting of suspected adverse drug reactions and for use of safety data to inform clinical care.
However, most low- and middle-income countries (LMICs) have weak regulatory capacities and lack functional systems to effectively monitor, mitigate, and avert adverse effects of medical products, endangering patients, the quality of care, and the achievement of desired health outcomes. This is especially a major risk as many new medicines have been introduced in LMICs at the same time as or instead of in high-income countries in recent years.
USAID MTaPS supports LMICs to build or strengthen PV systems and develop capacity to generate, analyze, and use safety data to improve health outcomes and the quality of care. The program implements the WHO Global Benchmarking Tool (GBT) as a guiding framework to help countries strengthen regulatory systems, including for establishing a functional PV system. This approach includes:
- Supporting countries to establish the legal and regulatory framework for PV
- Improving the demand for and supply and use of country-level safety data for clinical decision making
- Strengthening the processes for risk identification and characterization, risk assessment/evaluation, risk minimization, and safety communication
- Supporting the establishment of a system for active safety monitoring for novel and other high-risk medicines
A Functional Pharmacovigilance System
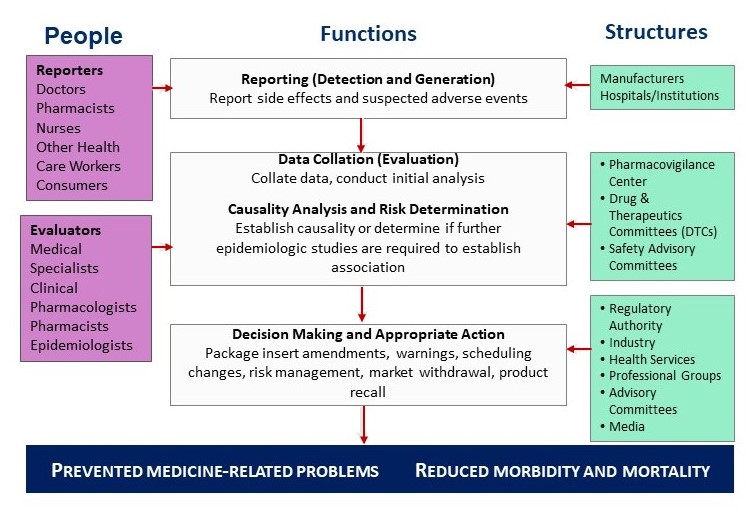
MTaPS’ support for countries in the area of PV includes:
- Establishing legal provisions, regulations, and guidelines
- Strengthening risk identification and characterization through increased reporting of suspected adverse drug reactions, feedback to providers, and the use of safety data to improve clinical decision making
- Providing tailored electronic data management solutions such as the web-based Pharmacovigilance Information Management System (PViMS) that support the effective collection and analysis of data from spontaneous and active surveillance systems (example, setting up pharmacovigilance for COVID-19 vaccines in Bangladesh)
- Advocating for reform to require increased participation of the pharmaceutical industry, distributors, and health facilities in PV at the country and regional levels
- Strengthening capacities for risk assessment and evaluation from spontaneous and active surveillance systems
- Minimizing risk through strategies to enforce regulatory decisions, updating of standard treatment guidelines and improving pharmacy practices based on results from surveillance findings
- Supporting participation in regional and global initiatives, such as WHO’s program for International Drug Monitoring implemented by the Uppsala Monitoring Centre
- Improving transparency, accountability, and communication across all stakeholders to raise awareness and strengthen PV systems and patient safety programs
Download Pharmacovigilance factsheet
For more information, contact:
Principal Technical Advisor, Pharmacovigilance
Alemayehu Duga
[email protected]

